Current Research
The research conducted in the Department of Molecular Biology mainly focuses on the activity of molecular chaperones in mammalian cells, including cell transformation. Using highly purified recombinant human proteins, we previously identified intermediate reactions that led to the assembly of molecular chaperone complexes with the wildtype or mutant p53 tumor suppressor protein. We also demonstrated that the heat shock protein 90 (HSP90) molecular chaperone was required for the binding of wildtype p53 to the promoter sequences under a physiological temperature of 37ºC and that chaperoning activity was adenosine triphosphate (ATP)-dependent. We recently provided in vivo evidence that HSP90 and HSP70/HSPA chaperone machines were required for the proper folding of wildtype p53, its specific binding to chromatin, and the transcription of p53-dependent genes (Walerych et al., Oncogene, 2009). We showed that molecular chaperones in human cells transfected with wildtype TP53, HSP90, and HSP70 maintained the native p53 conformation under heatshock conditions (42ºC) and assisted with refolding p53 at 37ºC during the recovery from heat shock. We also demonstrated that the interaction between wildtype p53 and the WAF1 promoter in cells was sensitive to HSP70 and HSP90 inhibition at 37ºC and further decreased upon heat shock. The influence of chaperones on the binding of p53 to the WAF1 promoter sequence was confirmed in vitro using highly purified proteins.
HSP90 stabilized the binding of p53 to the promoter sequence at 37ºC, whereas the requirement for the HSP70-HSP40 system and its cooperation with HSP90 increased under heat shock conditions. The Hop co-chaperone additionally stimulated these reactions. Interestingly, the combination of HSP90 and HSP70-HSP40 allowed for a limited in vitro restoration of DNA binding activity by the p53 oncogenic variant R249S and affected its conformation in cells. Our results indicated that both HSP90 and HSP70 were required for the chaperoning of wildtype and R249S p53, especially under stress conditions (Walerych et al., Oncogene, 2009).
We also elucidated the role of the adenine nucleotide in the HSP90 chaperone cycle by taking advantage of a unique in vitro assay that measures the HSP90-dependent binding of p53 to the promoter sequence (Walerych et al., J Biol Chem, 2010). E42A and D88N HSP90β variants bound but did not hydrolyze ATP, whereas E42A increased and D88N decreased ATP affinity compared with wildtype HSP90β. Nevertheless, both of these mutants interacted with wildtype p53 with similar affinity. Surprisingly, in the case of wildtype and E42A HSP90β, the presence of ATP stimulated the dissociation of HSP90-p53 complexes and resulted in the binding of p53 to the promoter sequence. D88N HSP90β is not efficient in either of these reactions. Using a trap version of the GroEL chaperonin, which irreversibly captures unfolded proteins, we showed that the action of the HSP90 chaperone on wildtype p53 resulted in a partial unfolding of the substrate. The ATP-dependent dissociation of the p53-HSP90 complex allowed further folding of the p53 protein to an active conformation that was able to bind to the promoter sequence. Further supporting these results, the overproduction of wildtype or E42A HSP90β stimulated transcription from the WAF1 gene promoter in H1299 cells. Altogether, our research indicated that the binding of ATP to HSP90β was a sufficient step for effective wildtype p53 client protein chaperoning (Walerych et al., J Biol Chem, 2010).
Heat shock protein 70 (HSP70/HSPA1) is an evolutionarily highly conserved molecular chaperone that promotes the survival of stressed cells by inhibiting lysosomal membrane permeabilization, a hallmark of stress-induced cell death. Clues to its molecular mechanism of action may lie in the recently reported stress- and cancer-associated translocation of a small portion of HSP70 to the lysosomal compartment. Prof. Marja Jaattela’s laboratory at the Denmark Cancer Institute, in collaboration with our department, showed that HSP70 stabilized lysosomes by binding to endolysosomal anionic phospholipid bis(monoacylglycero)phosphate (BMP), an essential cofactor for lysosomal sphingomyelin metabolism (Kirkegaard et al., Nature, 2010). In acidic environments, HSP70 binds with high affinity and specificity to BMP, thereby facilitating the BMP binding and activity of acid sphingomyelinase (ASM).
Inhibition of the HSP70-BMP interaction by BMP antibodies or a point mutation in HSP70 (Trp90Phe) and the pharmacological and genetic inhibition of ASM effectively reversed the HSP70-mediated stabilization of lysosomes. Notably, the reduced ASM activity in cells from patients with Niemann-Pick disease (NPD) A and B (i.e., severe lysosomal storage disorders caused by mutations in the sphingomyelin phosphodiesterase 1 [SMPD1] gene that encodes ASM) was also associated with a marked decrease in lysosomal stability, and this phenotype could be effectively corrected by treatment with recombinant HSP70. Altogether, these data open exciting possibilities for the development of new treatments for lysosomal storage disorders and cancer with compounds that enter the lysosomal lumen through the endocytic delivery pathway (Kirkegaard et al., Nature, 2010).
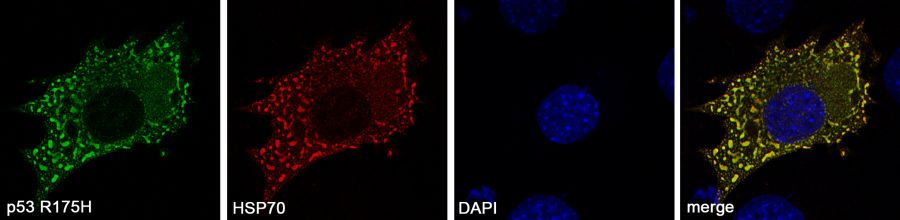
Fig. 1. Colocalization of overproduced HSP70 with mutated p53 R175H in MEF cells. MEF cell were transfected with plasmids encoding p53 R175H and HA-HSP70. 48 hours post-transfection the cells were fixed and stained with p53 (pseudocolour green) and HA tag (pseudocolour red) specifi c antibodies.
We discovered that MDM2, in addition to its E3-ubiquitin ligase activity, exhibited molecular chaperone activity and demonstrated that a MDM2 mutant protein that is defective in ATP binding (K454A) lacked chaperone activity both in vivo and in vitro. Wildtype MDM2 coexpressed with wildtype p53 stimulated effi cient p53 protein folding in vivo, and this effect was abrogated with an ATP binding-defective form of MDM2 (Wawrzynow et al., J Biol Chem, 2007).
Recently, in collaboration with Prof. Kathryn Ball at the University of Edinburgh, we showed that the binding affinity of MDM2’s hydrophobic pocket could be regulated through the RING finger domain and that increases in pocket affinity were reflected by a gain in MDM2 transrepressor activity (Wawrzynow et al., J Biol Chem, 2009). Thus, mutations within the RING domain that affect zinc coordination but not mutations that inhibit ATP binding produce MDM2 proteins that have a higher affinity for the BOX-I transactivation domain of p53 and a reduced affinity for p53 transrepression. An allosteric model of the regulation of the hydrophobic pocket was supported by differences in protein conformation and pocket accessibility between wildtype and RING domain mutant MDM2 proteins. Additionally, the data demonstrated that the complex relationship between different domains of MDM2 could impact the efficacy of anticancer drugs directed toward its hydrophobic pocket (Wawrzynow et al., J Biol Chem, 2009).
Numerous p53 missense mutations possess gain-of-function activities. Studies in mouse models have demonstrated that the stabilization of p53 R172H (R175H in humans) mutant protein by currently unknown factors is a prerequisite for its oncogenic gain-of-function phenotype, such as tumor progression and metastasis. Recently, we showed that the MDM2-dependent ubiquitination and degradation of p53 R175H mutant protein in mouse embryonic fibroblasts was partially inhibited by increasing concentration of HSP70/HSPA1-A. These phenomena correlated well with the appearance of HSP70-dependent folding intermediates in the form of dynamic cytoplasmic spots that contained aggregate-prone p53 R175H and several molecular chaperones (Fig. 1). We propose that a transient but recurrent interaction with HSP70 may lead to an increase in mutant p53 protein half-life. In the presence of MDM2, these pseudoaggregates can form stable amyloid-like structures that occasionally merge into an aggresome Interestingly, the formation of folding intermediates was not observed in the presence of HSC70/HSPA8, the dominant-negative K71S variant of HSP70, or an HSP70 inhibitor. In cancer cells, where endogenous HSP70 levels are already elevated, mutant p53 protein formed nuclear aggregates without the addition of exogenous HSP70. Aggregates that contained p53 were also visible under conditions in which p53 was partially unfolded (i.e., 37°C for temperature-sensitive variant p53 V143A and 42°C for wildtype p53). The refolding kinetics of p53 indicated that HSP70 caused transient exposure of the p53 aggregate-prone domains. We propose that the formation of HSP70- and MDM2-dependent protein coaggregates in tumors with high levels of these two proteins could be one of the mechanisms by which mutant p53 is stabilized.
Our results suggest that HSP70 molecular chaperone binds and partially unfolds p53. Upon ATP-dependent release of HSP70 from the complex with p53, part of the unfolded p53 protein, with the help of MDM2, is captured in the aggregationprone conformation (Fig. 2). Moreover, the sequestration of p73 tumor suppressor protein by these nuclear aggregates may lead to gain-of-function phenotypes (Wiech et al., PLoS One, 2012).
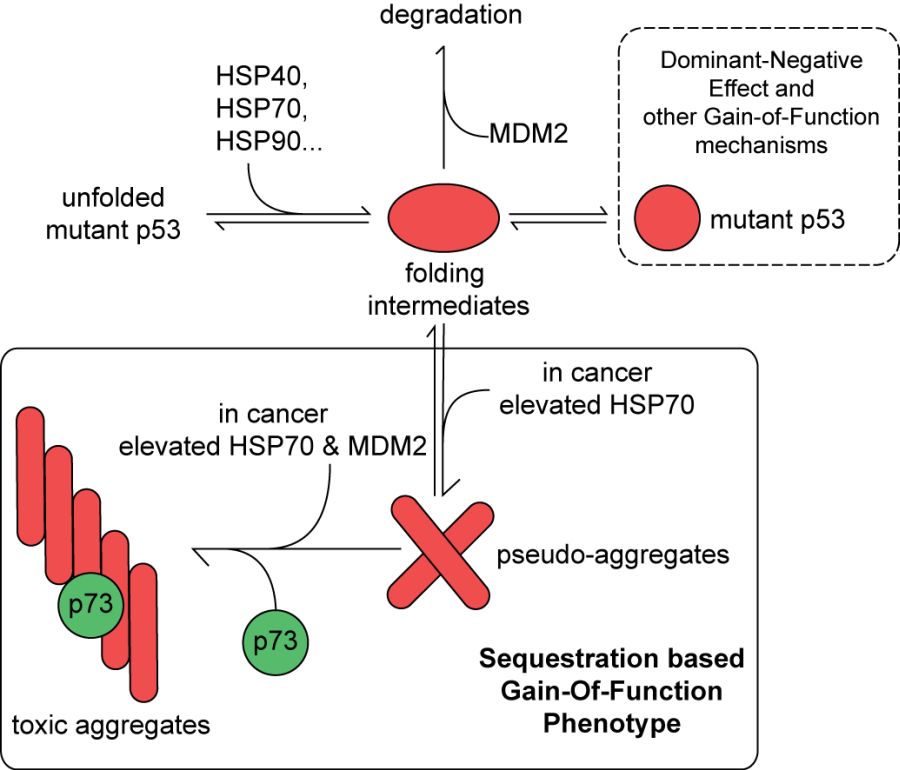
Fig. 2. Proposed model for stabilization of mutant p53 in cancer cells. Pro-oncogenic, gain-of-function phenotype of mutant p53 is governed by HSP70 and MDM2 levels. Recurrent interaction of HSP70 with the p53 polypeptide, in an ATP dependent manner, causes transient exposure of its aggregate prone domain(s). Subsequent aggregation of mutant p53 is further augmented by the MDM2-p53 allosteric interaction. This dynamic, irreversible molecular process can sequester other tumour suppressors, such as p73, thus inhibiting their activity. Pro-oncogenic activities of mutant p53 can be manifested through other, non-sequestration based mechanisms as depicted. We cannot exclude the possibility that molecular chaperones, including HSP70, are also involved in these processes.
Degrees:
1992 Professor, nomination by the President of the Republic of Poland
1986 DSc Habil in Molecular Biology, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland
1980 PhD in Biochemistry, Medical University of Gdansk, Poland
1977 MSc in Physics, University of Gdansk, Poland (student of physics and biology
Post-doctoral Training:
1982-1984 Department of Cellular, Viral and Molecular Biology, University of Utah, Salt Lake City, Utah, USA, and Department of Biochemistry, Stanford University, Stanford, California, USA
1979-1981 Department of Biochemistry, University of Gdansk, Poland
Professional Employment:
2005-Present President, Executive Director, Foundation for Polish Science
1999-Present Head, Department of Molecular Biology, IIMCB
1994-1999 Head, Department of Molecular and Cellular Biology, Faculty of Biotechnology, University of Gdansk, Poland
1991-1994 Head, Department of Molecular Biology, University of Gdansk, Poland
1993-1994 Visiting Professor, University of Utah, Medical Center, Institute of Oncology, Salt Lake City, Utah, USA
1990-1993 Vice President, University of Gdansk, Poland
1988-1991 Associate Professor, Department of Molecular Biology, University of Gdansk, Poland
1981-1988 Assistant Professor, Department of Biochemistry, University of Gdansk, Poland
Other Professional Activities:
2010-2015 Advisor of the President of the Republic of Poland
2010-2014 Member, ERC Identifi cation Committee (appointed by European Commission)
2010-2014 Chair of Selection Committee, Council of the National Science Center, Poland
2008-2010 Panel Chair, Molecular and Structural Biology and Biochemistry (LS1), ERC
2000-2004 Chair of Biology, Earth Sciences and Environmental Protection Commission, State Committee for Scientific Research, Poland
2000-2001 Chair of Basic Science Commission, State Committee for Scientifi c Research, Poland
Membership in Scientific Societies, Organizations, and Panels:
• European Molecular Biology Organization (EMBO), Member
• Polish Academy of Sciences, Full Member
• German National Academy of Sciences Leopoldina, Member
• Polish Academy of Arts and Sciences, Member
• Academia Europaea, Member
• European Academy of Cancer Research, Member
• American Society of Biochemistry and Molecular Biology, Member
• Advisory Editorial Board, EMBO Journal, EMBO Reports (2004-2008), and IUBMB Life, Member
• EMBO Council (2004-2007), Member
• Selection Committee, EMBO Young Investigator Programme (2001-2003), Member
• European Molecular Biology Conference (2001-2004), Polish delegate
• European Science Foundation Life Science Committee (2003-2005), Polish Delegate
• Selection Committee, Special DFG Programmes (2001-2005), Member
• Max Planck Society, Member of Senate (2012-Present)
• State Committee for Scientifi c Research (1997-2004), Member
Honors, Prizes, Awards:
2015 Commandor's Cross of the Order of Polonia Restituta
2013 Doctor Honoris Causa, Jagiellonian University
2011 Doctor Honoris Causa, University of Gdansk
2008 Offi cer’s Cross of the Order of Polonia Restituta (awarded by the President of the Republic of Poland)
2007 Doctor Honoris Causa, University of Wrocław
2002 Prime Minister Award for Scientifi c Achievements
2001 Marchlewski Award, Committee of Biochemistry and Biophysics, Polish Academy of Sciences
1999 Award in biological/medical sciences, Foundation for Polish Science
1996, 2007, 2010 Awards for best biochemistry work performed in Polish laboratories, Polish Biochemical Society
1994 Award from Ministry of Education
1993 Heweliusz Prize for Scientific Achievements (awarded by President of Gdansk)
1990 Award from Polish Academy of Sciences
1986 Individual Award for Scientific Achievements, Polish Academy of Sciences
Doctorates:
Liberek K, Skowyra D, Osipiuk J, Banecki B, Wojtkowiak D, Jakóbkiewicz J, Puzewicz J, Barski P, King F, Bućko-Justyna M, Kudła G, Helwak A, Lipiński L, Szymańska Z, Urbański J
Habilitations DSc Performed in Department:
K. Liberek, W. Werel, J. Marszałek , I. Konieczny , A. Wawrzynow , B. Banecki , P. Bieganowski.
Professor Titles Received:
K. Liberek, J. Marszałek, I. Konieczny, A. Wawrzynow.
Publications:Over 80 publications in primary scientifi c journals, including two papers published in Cell, six in EMBO J, six in Proc Natl Acad Sci USA, and more than 30 in J Biol Chem. These papers were cited more than 6200 times (including 23 papers cited more than 100 times)
Maciej Zylicz- past scientific accomplishments:
1. Isolation of the first Hsp70, the bacterial dnaK gene product, and discovery that Hsp70 possesses a weak ATPase activity that is stimulated by protein substrate (Zylicz et al., 1983, 249 citations). Subsequently, stimulation of Hsp70 ATPase activity in the presence of different protein and peptide substrates was demonstrated for all tested procaryotic and eucaryotic Hsp70s.
2. Isolation of the first Hsp40, the bacterial dnaJ gene product. Zylicz et al., 1985, 162 citations)
3. Isolation of the GrpE protein co-chaperone (Zylicz et al., 1986, 154 citations)
4. Role of DnaK and DnaJ heat shock proteins in the initiation of lambda DNA replication (Liberek et al., 1988-179 citations).
5. DnaK/DnaJ/GrpE chaperone machine dissociates the aggregated RNA Polymerase in an ATP-dependent reaction (Skowyra et al., 1990;-356 citations, Ziemienowicz et al., 1993,107 citations). Subsequently, the ability of Hsp70 chaperone machine to stimulate the dissociation of protein aggregates or more structured protein oligomers was demonstrated for all tested procaryotic and eucaryotic Hsp70s.
6. Reconstitution in vitro of the bacteriophage lambda DNA replication system (Zylicz et al., 1989, 247citations). For years this assay was utilized as a functional assay for purification of co-chaperones DnaJ and GrpE.
7. Purified bacterial DnaJ and GrpE protein efficiently stimulate DnaK ATPase activity (Liberek et al., 1991, 654 citations). DnaJ protein in this reaction accelerates the rate of ATP hydrolysis, whereas GrpE is a nucleotide exchange factor that stimulates the release of ADP from DnaK. Recently it was shown that functional interaction between DnaK and DnaJ is a paradigm for all tested procaryotic and eucaryotic systems.
8. Hsp40 and ATP hydrolysis activate Hsp70 for binding to different substrates (Wawrzynow et al., 1995, 199 citations). Similar mechanisms have also been observed for eucaryotic Hsc70 and Hsp40 (Misselwitz et al., 1998).
9. Discovery of a new subunit of AAA protease: ClpX (Wojtkowiak et al., 1994, 199 citations)
10. ATPase subunit of Clp protease possesses a chaperone activity (Wawrzynow et al., 1995, 200 citations). Similar results were independently obtained by two other laboratories (S. Lindquist and S. Wickner).
11. Discovery that wild type p53 tumor suppressor protein requires molecular chaperones for its antitumor activity (Zylicz et al., 2001, 143 citations; King et al., 2001, 104 citations; Walerych et al. 2004, 77 citations, Walerych et al., 2009, 24 citations)
12. Discovery that MDM2 oncogene possesses a molecular chaperone activity (Wawrzynow et. al., 2007, 33 citations)
Selected publications since 2001:
• Wiech M., Olszewski M.B., TraczGaszewska Z., Wawrzynow B., Zylicz M., Zylicz A. (2012) Molecular Mechanism of Mutant p53 Stabilization: The Role of HSP70 and MDM2, PLOS ONE 7(12) e51426
• Walerych D, Gutkowska M, Klejman MP, Wawrzynow B, Tracz Z, Wiech M, Zylicz M, Zylicz A. (2010) ATP binding to Hsp90 is sufficient for effective chaperoning of p53 protein. J Biol Chem 285, 32020-32028
• Zubrienė A, Gutkowska M, Matulienė J, Chaleckis R, Michailovienė V, Voroncova A, Venclovas C, Zylicz A, Zylicz M, Matulis D. (2010) Thermodynamics of radicicol binding to human Hsp90 alpha and beta isoforms. Biophys Chem, 152, 153-163
• Walerych D., Olszewski M., Gutkowska M., Helwak A., Zylicz M., Zylicz A., (2009) Hsp70 molecular chaperones are required to support p53 tumour suppressor activity under stress conditions. Oncogene 28, 4284-4294
• Narayan V, Eckert M, Zylicz A, Zylicz M, Ball LK. (2009) Cooperative regulation of the IRF-1 tumour suppressor protein by core components of the molecular chaperone machinery. J Biol Chem, 284- 25889-99
• Szymanska Z, Zylicz M. (2009) Mathematical modeling of heat shock proteins synthesis in response to temprerature change. J Math Biol, 58: 819-844
• Wawrzynow B., Zylicz A., Wallace M., Hupp T., Zylicz M. MDM2 Chaperones the p53 Tumor Suppressor. J Biol Chem. (2007) 282:32603-12
• Kudla G, Lipinski L, Caffin F, Helwak A, Zylicz M (2006) High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 4: 0933-42
• Jassem J, Jassem E, Jakobkiewicz-Banecka J, Rzyman W, Badzio A, Dziadziuszko R, Kobierska-Gulinda G, Szymanowska A, Skrzypski M, Zylicz M (2004) P53 and K-ras mutations are frequent events in microscopically negative surgical margins from patients with non-small cell lung carcinoma. Cancer, 100: 1951-1960
• Dworakowska D, Jassem E, Jassem J, Peters B, Dziadziuszko R, Zylicz M, Jakobkiewicz-Banecka J, Kobierska-Gulida G, Szymanowska A, Skokowski J, Roessner A, Schneider-Stock R (2004) MDM2 gene amplification: a new independent factor of adverse prognosis in non-small cell lung cancer (NSCLC). Lung Cancer 43: 285-295
• Walerych D, Kudla G, Gutkowska M, Wawrzynow B, Muller L, King FW, Helwak A, Boros J, Zylicz A, Zylicz M (2004) Hsp90 Chaperones Wild-type p53 Tumor Suppressor Protein. J. Biol. Chem., 279: 48836-48845
• Jassem E, Niklinski J, Rosell R, Niklinska W, Jakobkiewicz J, Monzo M, Chyczewski L, Kobierska G, Skokowski J, Zylicz M, Jassem J (2001) Types and localisation of p53 gene mutations. A report on 332 non-small cell lung cancer patients. Lung Cancer, 34: 47-51
• Zylicz M, Wawrzynow A (2001) Insights into the function of Hsp70 chaperones. IUBMB, 51: 283-287
• King FW, Wawrzynow A, Hohfeld J, Zylicz M (2001) Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 20: 6297-6305
• Zylicz M, King FW, Wawrzynow A (2001) Hsp70 interactions with the p53 tumour suppressor protein. EMBO J. 20: 4634-4638
• Genevaux P, Wawrzynow A, Zylicz M, Georgopoulos C, Kelley WL (2001) DjlA is a Third DnaK Co-chaperone of Escherichia coli, and Dj1A-mediated Induction of Colanic Acid Capsule Requires Dj1A-DnaK Interaction. J. Biol. Chem. 276: 7906-7912
• Banecki B, Wawrzynow A, Puzewicz J, Georgopoulos C, Zylicz M (2001) Structure–function analysis of the zincbinding region of the ClpX molecular chaperone. J. Biol. Chem. 276: 18843-18848



