 |
Jacek Jaworski, PhD, Professor
Correspondence address:
Laboratory of Molecular and Cellular Neurobiology
International Institute of Molecular and Cell Biology
4 Ks. Trojdena Street, 02-109 Warsaw, Poland
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
tel: +48 (22) 597 0755; fax: +48 (22) 597 0715
|
DEGREES
2014 - Professor of Biological Sciences, nomination by the President of the Republic of Poland
2010 - DSc Habil in Molecular Biology, Warsaw University, Poland
2001 - PhD in Molecular Neurobiology, Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland
1996 - MSc in Biology, Department of Genetics, Warsaw University, Poland
PROFESSIONAL EXPERIENCE
2018-present - Deputy Director for Science, International Institute of Molecular and Cell Biology in Warsaw, Poland
2010 - 2013 - Deputy Director, International Institute of Molecular and Cell Biology in Warsaw, Poland
2005-present - Professor, Head of Laboratory of Molecular and Cellular Neurobiology, International Institute of Molecular and Cell Biology in Warsaw, Poland
RESEARCH TRAINING
2016 - Research visit (3 weeks) with Prof. William Harris, Cambridge University, Cambridge, UK
2011 - Research visit (2 weeks) with Dr. Carlo Sala, CNR Institute of Neuroscience and Instituto Neurologico Carlo Besta, Milan, Italy
2006 - Research visit (1 month) with Dr. C.C. Hoogenraad, Erasmus Medical Center, Rotterdam, Holland
2002-2005 - Postdoctoral Associate with Prof. Morgan Sheng, Picower Center for Learning and Memory, Massachusetts Institute of Technology and Howard Hughes Medical Institute, Cambridge, MA, USA
2000 - Research training with Dr. J. Guzowski, ARL Division of Neural Systems, Memory and Aging, University of Arizona, Tucson, USA
1997-2001 - Research training (7 months) with Prof. J. Mallet, Laboratoire de Genetique Moleculaire de la Neurotransmission et des Processus Neurodegeneratifs (LGN), UMR 9923 CNRS, Paris, France
1996-2002 - PhD student (until 2001) and Postdoctoral Associate (until May 2002) with Prof. L. Kaczmarek, Laboratory of Molecular Neurobiology, Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland
1995-1996 - Master’s degree, Prof. P. Węgleński, Department of Genetics, Warsaw University, Poland
FELLOWSHIPS AND AWARDS
2018 - TEAM, Foundation for Polish Science
2014 - Master Award, Foundation for Polish Science
2011 - Prime Minister Award for habilitation thesis
2009 - 2nd Division (Biological Sciences) of Polish Academy of Sciences Award for series of publications on MMP9 (together with teams of Prof. Kaczmarek and Dr. Wilczynski)
2005 - Konorski Award for best publication of 2004 in the field of neuroscience (Kowalczyk et al., JCB, 2004, 167:209-213), Polish Neuroscience Society and Polish Academy of Sciences
2002 - Prime Minister Award for PhD thesis
2001 - Foundation for Polish Science National Scholarship for Young Investigators (1 year scholarship)
2000 - EMBO Short Term Fellowship
1999 - Polish Network for Cell and Molecular Biology UNESCO/PAN Scholarship
1997 - Bourse de Stage du Gouvernement Francaise (French Government Scholarship)
MEMBERSHIP IN SCIENTIFIC SOCIETIES, ORGANIZATIONS, AND PANELS
2019 - Member, Scientific Advisory Board of the Institute of Pharmacology, Polish Academy of Sciences
2017 - Vice President, Polish Neuroscience Society (term 2017-2019)
2015 - Corresponding Member, Warsaw Scientific Society
2015 - Member, Scientific Advisory Board of the Nencki Institute of Experimental Biology, Polish Academy of Sciences
2011 - Member, Neurobiology Committee, Polish Academy of Sciences (terms 2011-2014; 2015-2018; 2019-2020)
DOCTORATES DEFENDED UNDER LAB LEADER’S SUPERVISION
Ł. Świech, A. Malik, M. Perycz, M. Urbańska, A. Skałecka, J. Lipka, A. Urbańska, M. Firkowska, K. Kisielewska, A. Kościelny.
DESCRIPTION OF CURRENT RESEARCH
Mammalian/mechanistic target of rapamycin (mTOR) is a serine-threonine kinase that is involved in almost every aspect of mammalian cell function. It forms two protein complexes, initially identified as regulating translation (mTOR complex 1 [mTORC1]) or influencing the actin cytoskeleton (mTORC2). My postdoctoral work showed that the regulation of mTOR-dependent translation contributes to dendritogenesis (Jaworski et al., J Neurosci, 2005). This was subsequently confirmed by our recent work in which we identified the GluA2 subunit of glutamate receptors as a protein that is both translated in an mTORC1-dependent manner and vital for dendritogenesis (Koscielny et al., Mol Neurobiol, 2018). However, the list of cellular processes that involve both mTORCs has expanded, and new ways of regulating mTORC activity, novel mTOR partners, and mTOR effectors have been discovered. Nonetheless, their contribution to the neuronal functions of mTOR and neuropathology is still poorly understood. Therefore, since the inception of our laboratory, we have sought to identify mTOR partners and regulated proteins that are involved in neuronal development and characterize mTOR dysfunction in neuropathology.
To reach our scientific objectives, we have been primarily using a well-established, relatively simple, and robust model of the dendritogenesis of neurons that are cultured in vitro. Using this approach, we performed both proof-of-principle experiments and unbiased screens that clearly demonstrated mTOR functions during neuronal development beyond the canonical control of translation (e.g., regulation of the cytoskeleton and transcription). These experiments also extended our general knowledge of molecular mechanisms downstream of mTOR and new mechanisms that underlie dendritogenesis (Swiech et al., J Neurosci, 2011; Urbanska et al., J Biol Chem, 2012 & Sci Rep, 2017; Malik et al., J Biol Chem, 2013).
Progress in meeting our research goals allowed us to merge some objectives and hone our main focus toward identification of the cellular compartment-specific regulation and functions of mTOR in developing neurons, with a particular focus on intracellular trafficking events, which were at the center of our research efforts during the last 6 years (Main Research Objective 1). Notably, both the role of mTOR in intracellular trafficking control and the role of membrane trafficking in neuronal development and disease are still understudied topics.
Therefore, focusing on these areas (e.g., the interplay between mTORCs and molecular motors, such as the dynein-dynactin complex and kinesins, and small GTPases of the Rab family and their regulators) creates an opportunity to successfully proceed with our research in otherwise extremely crowded fields of the molecular biology of mTOR and mTOR-related disorders.
An important part of our work during the last 6 years has been to develop and characterize new approaches to study mTOR functions in vivo beyond dendritogenesis (i.e., in utero brain electroporation in rodents and transgenic zebrafish) and in clinically relevant material (e.g., patient samples, primary cultures, induced pluripotent stem cells, and organoids). These modern techniques, together with newly identified mTOR-controlled molecular processes, are critically important for our second main objective, namely understanding the molecular pathology of mTORopathies (Main Research Objective 2), which are diseases that are related to mTOR dysregulation (e.g., tuberous sclerosis complex (TSC) and epilepsy).
By studying mTOR in the context of the control of dendritic arbor morphology, we identified a significant gap in the literature about this phenomenon. Dendritic arbor morphology is unique for different types of neurons and reflects their precise adjustment to functions they perform within particular neuronal networks. Although dendrites must remain intact for more than 80% of a neuron’s lifespan, little is known about the molecular mechanisms that underlie this phenomenon. To date, very few proteins have been identified to be essential for the stability of mature dendritic arbors. Disturbances in dendritic arbor stability in the mature brain are related to prolonged stress and mood disorders (e.g., depression). At later stages of brain aging, when cognitive decline develops, dendrites may also deteriorate. Intriguingly, recent studies reported changes in mTOR signaling in mood disorders and aging. Thus, our new Main Research Objective 3 seeks to understand the molecular mechanisms of dendrite stability and their disruption in mood disorders and the aging brain.
Our major success in 2019 falls within Main Research Objective 2, which focuses on mTORrelated disorders. One such disease is TSC. It is a multiorgan disease that is caused by mutations of the TSC1 and TSC2 genes, the products of which form a complex (TSC complex) that inhibits mTORC1 (Switon et al., IUBMB Life, 2016). The most common symptoms of TSC are epilepsy, autism, the formation of benign tumors in the brain, and socalled TSC-associated neuropsychiatric disorders (TANDs). We used zebrafish with the mutated Tsc2 gene to study causal links between elementary neurodevelopment and epilepsy/TANDs. We found that the lack of Tsc2 in zebrafish resulted in heterotopias (Fig. 1A) and hyperactivation of the mTorC1 pathway in pallial regions, which are homologous to the mammalian cortex (Kedra et al., Proc Natl Acad Sci USA, 2020). We observed commissural thinning (Fig. 1B) that was responsible for brain dysconnectivity, recapitulating TSC pathology in humans (Kedra et al., Proc Natl Acad Sci USA, 2020). The mutants exhibited epileptogenesis that resulted in nonmotor seizures and an increase in anxiety-like behavior (i.e., one symptom of TANDs; Kedra et al., 2020; Fig. 1C,D), which were rescued by reducing tyrosine receptor kinase B (TrkB) signaling. TrkB inhibition also rescued brain dysconnectivity. These data provide a mechanistic link between brain anatomy and human TANDs and identify TrkB as a possible lead target in the search for TAND-specific drugs.
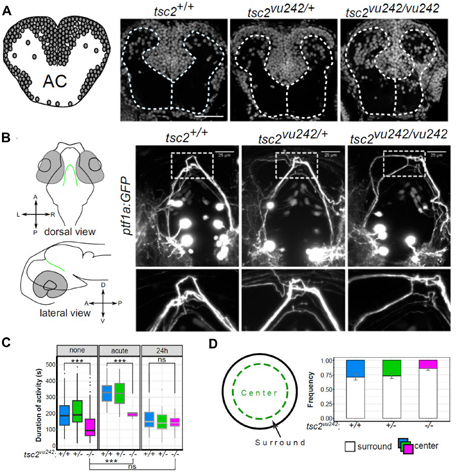
fig.1. Zebrafish model of Tuberous Sclerosis Complex (tsc2vu242/vu242) can be used to study several disease symptoms. (A) Visualization of cell nuclei using DAPI shows that in TSC fish brain several cells are mislocalized causing disruption of white matter. AC - anterior commissure (B) ptf1a:GFP-positive neurons in the posterior tuberculum extend their axons dorsally and cross the brain midline through the posterior commissure (left panel). In the TSC fish these axonal tracts are disorganized and do not cross the midline as compact bundle (right panel). (C) TSC fish display absence seizure-like phenotype that can be “cured” with prolonged ethosuximide treatment used typically in clinic. (D) In open field test TSC fishes avoid center of the dish suggesting increased anxiety. For more details, see Kedra et al., Proc Natl Acad Sci USA, 2020. Photo: Justyna Zmorzyńska, PhD and Magdalena Kędra, MSc.

Lab Leader:
Senior Researchers:
-
Ewa Liszewska, PhD
-
Matylda Macias, PhD (part-time)
-
Małgorzata Urbańska, PhD
-
Justyna Zmorzyńska, PhD
-
Agnieszka Brzozowska, PhD
Postdoctoral Researchers:
-
Andrii Kopach, PhD
-
Aleksandra Tempes, PhD
-
Tomasz Dulski, PhD
Research Assistant:
Research Technicians:
PhD Students:
-
Magdalena Kędra, MSc
-
Magdalena Mlostek, MSc
-
Karolina Bogusz, MSc
-
Oliver Tkaczyk, MSc
-
Jan Węsławski, MSc
-
Juan Zeng, MSc
-
Olga Doszyń, MSc
Lab Technician:
Laboratory Support Specialist:



